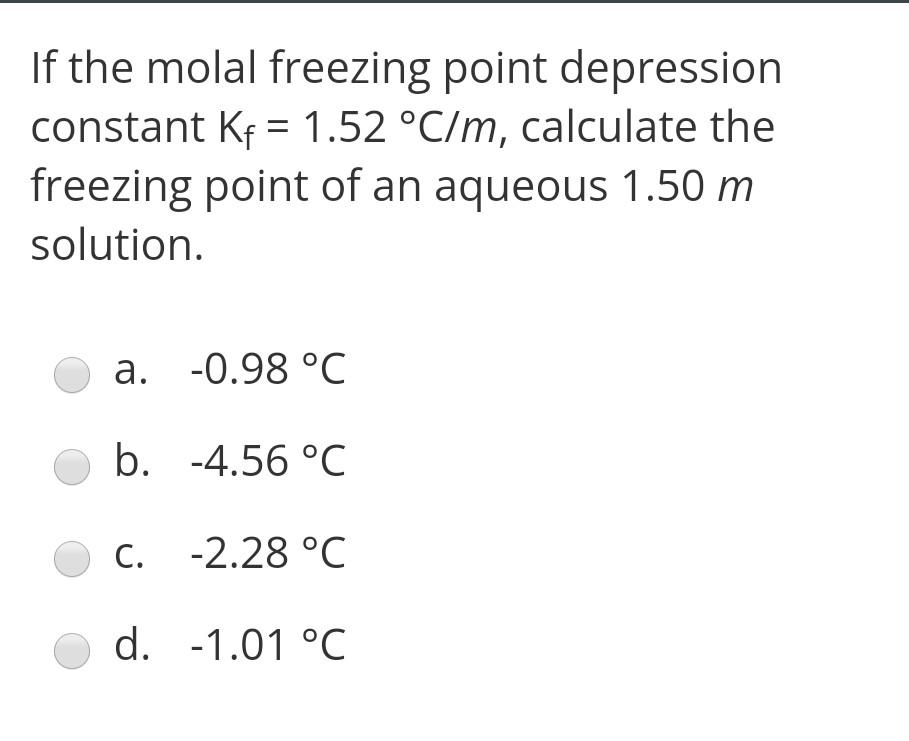
After you preform your experiment, you determine that the Kf value for naphthalene is 6.9 . You are using 10g of naphthalene and added 1.0 g of your unknown. The the freezing

Calculate Complex Ion Equilibria Using the Small x Approximation for Large Kf | Chemistry | Study.com

Calculate the freezing point of a solution when 3 g of CaCl2(M = 111 g mol^-1) was dissolved in 100 g of water.assuming CaCl2 Undergoes complete ionisation (Kf for water = 1.86 K kg mol^-1)

After you preform your experiment, you determine that the Kf value for naphthalene is 6.9 . You are using 10g of naphthalene and added 1.0 g of your unknown. The the freezing

0.15 molal solution of NaCI has freezing point -0.52 ^(@)C Calculate van't Hoff factor .(K(f) = 1.86 K kg mol^(-1) )

Calculate the amount of KCl which must be added to 1 kg of water so that the freezing point is depressed by 2K. (kf for water = 1.86 K kg mol^-1)